In 2017 I wrote a very enthusiastic blog post about a new biologic agent for atopic dermatitis. After six years of treatment,
I’m still quite enthusiastic and recommend Dupixent to patients, friends, and family. I've put the post below along with my comments (in parentheses) on my current experience.
Although this post is most unskeptical and decidedly noncardiac, the skeptical cardiologist feels compelled to share this information with readers who have or know friends or family with eczema or atopic dermatitis, a chronic skin condition that results in itchy, scaly, dry, and red skin.
For most of my life, I have dealt with periodic flare-ups of eczema along with continuously itchy skin. Control of flare-ups was by meticulous attention to keeping my skin clean and moisturized along with frequent applications of topical corticosteroids.
Things worsened a few years ago and I began to think I might have Red Skin Syndrome which some dermatologists believe is due to withdrawal from topical corticosteroids.
Two months ago, however, my spirits brightened when I heard that the FDA had approved a new biologic injectable called Dupixent (dupilumab), to treat adults with moderate-to-severe eczema (atopic dermatitis), whose eczema is not controlled adequately by topical steroids.
My fantastic dermatologist, Dr. Amy Ney, agreed this was appropriate therapy for me, and within a week I received a refrigerated package containing the initial dosage: two syringes filled with the drug.

Within a week of injecting the contents of the syringes into my abdomen, my itching ceased and I had no more eczematous rashes. For me, this was a minor miracle.
Since then I've injected one 300 mg syringe every two weeks and I continue to be free of my life-long signs and symptoms of eczema.
(As of August, 2023 I have reduced the frequency to around every 2 months.)
Atopic Dermatitis and Dupixent
The cause of atopic dermatitis is a combination of genetic, immune, and environmental factors. In atopic dermatitis, the skin develops red, scaly and crusted bumps, which are extremely itchy. Scratching leads to swelling, cracking, “weeping” clear fluid, and finally, coarsening and thickening of the skin.
Dupixent’s active ingredient is an antibody (dupilumab) that binds to a protein [interleukin-4 (IL-4) receptor alpha subunit (IL-4Ra)], that causes inflammation. By binding to this protein, Dupixent is able to inhibit the inflammatory response that plays a role in the development of atopic dermatitis.
Dupixent acts by inhibiting two cytokines that are responsible for the hyperimmune response in skin. They are called IL-4 and IL-13. IL is an abbreviation for interleukins, proteins that are produced by leukocytes (3) and play a part in regulation of the immune system. Steroids, such as prednisone, also suppress the immune system, but taking them for an extended period of time will get you into trouble. (See: Prednisone: Satan's Little Helper) Unlike prednisone, Dupixent inhibits specific targets. It works more like a scalpel than a bomb.
More Stories of Miraculous Relief
I am not alone in experiencing miraculous relief from this new drug. I first heard of it from a New York Times article in 2016 which details the dramatic responses of several patients who were involved in the clinical trials that proved the drug's efficacy:
One participant in the trial, Lisa Tannebaum, a 53-year-old harpist in Stamford, Conn., was so thrilled that she wrote a letter to Regeneron suggesting they use her before and after photographs in advertisements. She developed a severe form of the disease 14 years ago and tried everything imaginable in conventional and alternative medicine without relief — specialized diets, immunosuppressive drugs, special clothing, bleach baths. She even had the gold fillings removed from her teeth on the theory that they may be causing an allergic response, but to no avail.
“It was like every day I had poison ivy and fire ants on myself,” she said. “You don’t sleep at all. You can’t go out, you have staph infections all the time,” because the skin’s protective barrier is broken by the rash. “I couldn’t drive my kids to school because the itching was so bad I couldn’t put my hands on the steering wheel.”
Now, she is performing again and will be playing her harp at Carnegie Hall on Oct. 30.
Randomized Controlled Trials Proving Efficacy
Of course we can't rely on anecdotes to prove the safety and efficacy of drugs: we need randomized, controlled, double-blind studies.
Dupixent has three such clinical trials with a total of 2,119 adult participants, and the results were remarkable. (for details see here). Overall, participants who received Dupixent achieved greater response, defined as clear or almost clear skin, and experienced a reduction in itch after 16 weeks of treatment.
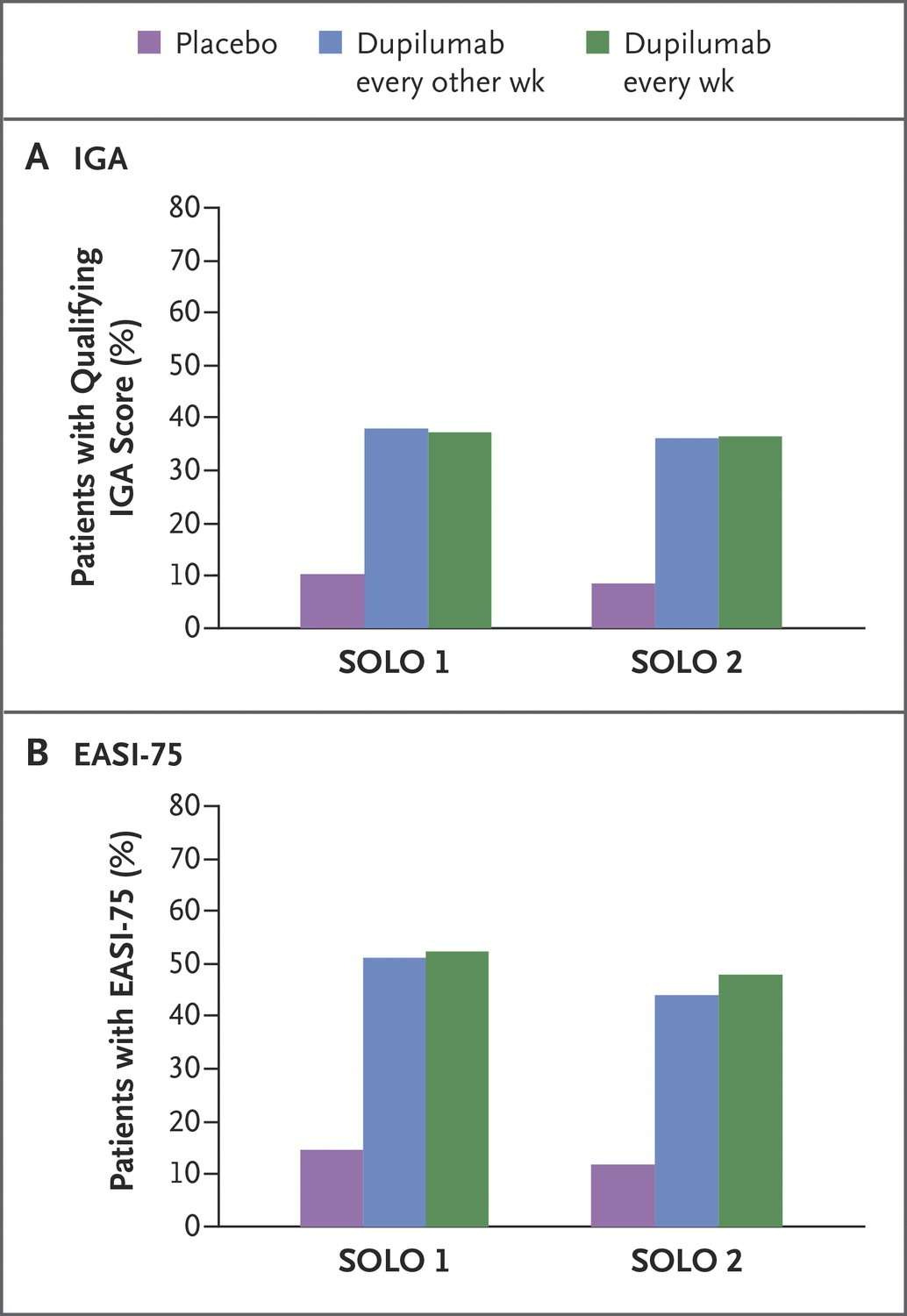
The only side effect which was more common with Dupixent than placebo was conjunctivitis, an inflammation of the eye.
(I have experienced significant worsening of my allergic conjunctivitis since starting Dupixent. I've read everything I could on this topic and tried everything from Restasis to steroid eye drops. It remains an issue but for me (and for most who go on Dupixent) the relief from eczema is so wonderful that we continue the drug despite the eye issues.)
Cost of Dupixent
When I first read of this drug I assumed it would be horribly expensive. In cardiology we have two injectable biologics (Repatha and Praluent, PCSK9 inhibitors) for lowering cholesterol, which typically have been costing my patients with insurance coverage over 1000$ per month.
Fortunately, Sanofi/Regeneron have learned from prior experience and priced the drug at $37,000, a number that insurance companies have apparently warmly welcomed. This article suggests that the drug is priced significantly lower than newer biologics now available for psoriasis and rheumatoid arthritis.
With my insurance (United Health Care) coverage, I was asked to pay 150$ for 2 injections per month. I then discovered that Sanofi has a co-pay card that covers that 150$ so for now I am paying zero dollars out of the 37,000$.
I'm paying nothing for a brand-new biologic injectable that has cured my eczema. Now that is miraculous!
(As of 2023, when I transitioned to Medicare, despite a good supplemental medication plan, my out-of-pocket cost for Dupixent has increased to >$5000. The co-pay card only works with private insurance. Hopefully, this will change as competitors emerge or with the expiration of the drug's patent.)
Unskeptically Yours,
-ACP
N.B. Featured Image before and after hand is from National Eczema organization and is not of my hand.





Might diet play a role? The original randomized, double-blind, controlled trial of diet and eczema found that cutting out eggs, chicken, milk, and beef significantly improved eczema in 70 percent of the kids who completed the study. Subsequent studies found similar results. In fact, out of 13 studies on avoiding milk, eggs, or both, 10 studies documented overall clinical improvement. Might be worth a try since the side effects of a mostly plant based diet are all beneficial. Not so for dupixent.
Ah, dermatology. I've heard it said about my colleagues the periodontists that they're like dermatologists. Their patients seldom die, and they seldom get well. Glad you were able to put the lie to that one.
Now Dupixent needs a famous spokesperson like Cyndi Lauper for Cosentyx--"Five yeuhs cleuh!"